Home
electronic Self-Administered Eczema Area and Severity Index
eSA-EASI
Eczema self-assessment made
easy and quick
Illustrative PDF
(coming soon)
Original PDF
(coming soon)
What is the eSA-EASI?
The electronic Self-Administered Eczema Area and Severity Index (eSA-EASI) allows the patient or caregiver to assess the severity of atopic dermatitis by themselves. A regular SA-EASI assessment may help to continuously monitor the progression of the disease and determine the effect of a treatment.
Validation of questionnaire
paper version: yes digital version: no
How to use the eSA-EASI
The SA-EASI is a measurement tool for self-assessment that can be filled out by the patient or the caregiver. Below is an example of the eSA-EASI assessment process in two steps.
First, in the paper version of the SA-EASI a line-drawing silhouette of the back and front is presented to shade in the atopic dermatitis affected body area. In the electronic version of the SA-EASI, the body surface area (BSA) is calculated using the patient's palm, with one palm representing approximately 1 % of the BSA.

Second, five visual analog scales (VAS), enable patients or caregivers to describe the redness, thickness, dryness, itchiness, and scratchiness of a typical lesion.
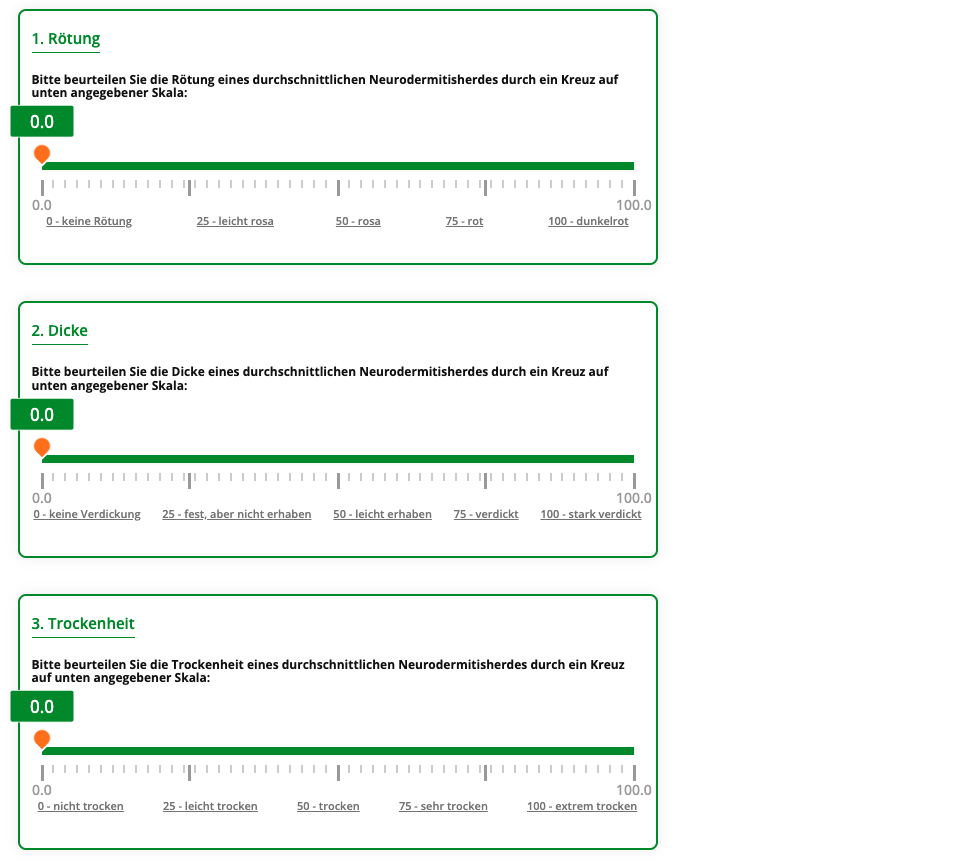
To calculate the SA-EASI, the affected body surface area (BSA) is multiplied by the sum of the values of the visual analogue scales (redness, dryness, thickness, scratchiness). The DermaValue web and smartphone application calculates the SA-EASI value automatically. In addition, the acute and chronic SA-EASI as well as the total affected BSA are also displayed.
Affected BSA (0 - 6) = number of palms / 100 * 6
Acute SA-EASI (0 - 72) = sum of VAS (thickness + redness + scratchiness) * 0,04 * BSA
Chronic SA-EASI (0 - 24) = VAS dryness * 0,04 * BSA
Total SA-EASI (0 - 96) = chronic SA-EASI + acute SA-EASI
The following versions of the eSA-EASI are available
Illustrative PDF
(coming soon)
Original PDF
(coming soon)
How was the eSA-EASI developed?
The SA-EASI was developed at the Bowman Gray School of Medicine of Wake Forest University in the USA. The SA-EASI is a modified version of the original EASI, which requires trained staff for the measurement. However, whereas in the original version the physician is asked to assign the severity of the disease on a scale from 0 – 3 for each body part, the severity of an average lesion is marked by patients on visual analog scales (0 – 100) in the SA-EASI. Instead of the calculation of the involved Body Surface Area (BSA) by trained personnel, a line-drawing silhouette of the front and back is marked by the patient to estimate the involved body area. In the electronic version of the SAPASI, the body surface area is calculated using the patient's palm, with one palm representing approximately 1 % of the body surface area. Since its original development, the SA-EASI was validated in multiple studies and showed good agreement with the original and well accepted EASI. The electronic version of the SA-EASI is currently validated at the Institute for Health Services Research in Dermatology and Nursing at the University Medical Center Hamburg-Eppendorf.
The impact of eczema
Eczema is a skin condition characterized by patches of inflamed, itchy, red, cracked, and swollen skin. The term "eczema" and "dermatitis" are often used interchangeably, with the most commonly condition being atopic eczema/dermatitis. Atopic eczema is an inflammation of the dermal layer of the skin resulting in inflamed, red, sometimes blistered, and rough patches of skin.
The condition is chronic, prone to relapse, and often extremely pruritic. While the condition may occur at any age, it is most common in childhood and severity varies throughout the life cycle. The exact cause is unclear, but it is known to result from a combination of genetic (hereditary) and environmental factors.
Atopic eczema has a severely negative effect on quality of life (QoL), with patients reporting skin damage, soreness, sleep deprivation (due to itching), activity restriction and depression. The lifestyle burden cannot be underestimated with frequent doctor visits, special clothing and the need to constantly apply messy topical ointments adding to the patient distress. Children with severe cases tend to have a smaller social circle and participate in fewer group activities than their peers.
They are also at higher risk for developing depression, anxiety, and other mental health disorders. The burden is also carried by parents, who report of a lack of sleep and privacy (often due to co-sleeping), treatment-related financial expenditures, and feelings of hopelessness, guilt, and depression. It's estimated that parents of children with moderate to severe atopic eczema spend up to 3 hours a day caring for their children's skin.
Features
PATIENT-CENTERED
Results from the eSA-EASI provide a patient-orientated analysis of atopic dermatitis severity.
QUICK
Save time with our online tool. Fill in the questionnaire and your eSA-EASI will be calculated automatically.
USER-FRIENDLY
The eSA-EASI is easy and intuitive to use.
VALIDATED
Since its original development the SA-EASI was validated in multiple studies and showed good agreement with the original and well accepted EASI.
MONITOR PROGRESS
The eSA-EASI can be used to monitor the change in atopic dermatitis severity over the course of treatment.
ACCESSIBLE FROM ALL DEVICES
With DermaValue, your eSA-EASI is always just a few clicks away. The application is available for PCs, tablets, smartphones, and other devices.
References
Validity study:
Housman, T. S., Patel, M. J., Camacho, F., Feldman, S. R., Fleischer, A. B., Jr, & Balkrishnan, R. (2002). Use of the Self-Administered Eczema Area and Severity Index by parent caregivers: results of a validation study. The British journal of dermatology, 147(6), 1192–1198. https://doi.org/10.1046/j.1365-2133.2002.05031.x
Our other tools
Our partners





